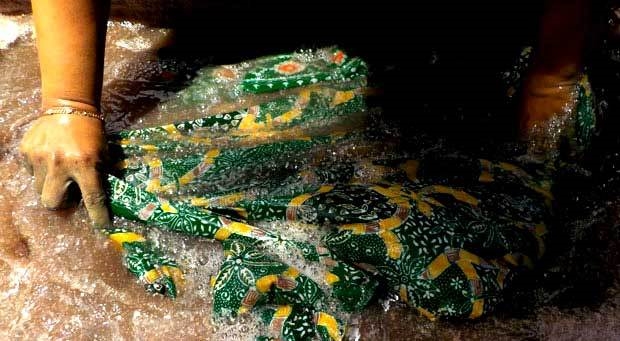
Water is one of the basic needs of all living things in this world. However, there are many infeasible water sources used, even though they are polluted. One of the causes of pollution in water is the amount of disposed wastewater from various types of industries without undergoing water management as regulated in environmental quality standards.
The government has regulated water pollution control through Government Regulation Number 82 of 2001. There is also a Regulation of Minister of Environment Number 5 of 2014 which regulates the quality standards of wastewater disposable to the environment.
There are some general parameters determined as standard values of quality and characteristics of wastewater such as BOD (Biochemical Oxygen Demand), COD (Chemical Oxygen Demand), and DO (Dissolved Oxygen). BOD is the amount of oxygen needed by bacteria to break down organic matter in wastewater while COD is the total amount of oxygen needed to oxidize organic matter chemically, and DO shows the oxygen content in water.
Batik industry is one of the domestic industries which develop very well. As one of the reliable export commodities, this industry has brought many benefits to the country. But unfortunately, the rapid development of batik industry also caused environmental problems from the dyes used in production and disposed of wastewater.
Although some regions of the batik production center have made efforts for waste management to overcome and prevent contaminated water from batik production, they have not been optimal to reduce water pollution in the environment.
Synthetic chemical dyes such as azo dye are widely used in the batik industry. Azo dyes are difficult to decompose because they have an azo functional group attached to an aromatic ring, which is difficult to degrade in the environment. Furthermore, azo dye is also carcinogenic, which can threaten the life of living creatures due to continuous exposure.
In general, batik washing water produced by batik home industry is dense in color and is not easily broken down. It contaminated the rivers as the batik industry wastewater contain high organic matter and fat content.
The innovation of batik wastewater treatment gets a lot of attention because batik wastewater contains dyes that are difficult to remove. The electrocoagulation method is one of the innovations to process colorful wastewater from the batik industry. In principle, the electrocoagulation method is carried out on a reactor containing wastewater which is equipped with two electrodes as an anode and cathode.
Furthermore, a unidirectional electric current is used to flow the two electrodes so that electrochemical events occur. Reduction and oxidation reaction occur at the electrodes. During electrification, positive ions moving to the cathode and receiving electrons that are reduced and negative ions are moving to the anode and giving up the oxidized electrons.
In this process, a floc will eventually form due to the reaction between the anode and the contaminants in the wastewater and the resulting floc can be set aside. Electrocoagulation process has some advantages such as easy operation and short removal process time.
Batik waste treatment research using several variations of metal types as electrodes have been carried out. The combination of aluminum, iron, and stainless steel is used in the electrocoagulation process of a reactor. The choice of metal for electrodes is very important in the electrocoagulation process because it is related to the efficiency of waste removal and durability of the metal during the electrocoagulation process. In addition to the type of metal electrode, the height of the electrode immersed in the waste, the distance between the electrodes, and the operating time are affecting parameters.
Experiments on batik waste processing showed that the combination of metal types used as electrode material produced the different final color reduction results in batik wastewater. The use of aluminum metal as anode and ferrous metal as a cathode with full immersion for 50 minutes resulted in color reduction efficiency in batik wastewater of 96.45%. There was a 50-60% decrease in COD and discoloration from the compared water before and after batik wastewater treatment.
For other types of metal combinations, the combination of aluminum and iron as electrodes affect the color greater than the combination of aluminum and stainless steel but lower than the combination of aluminum and iron. The results of this research on batik wastewater treatment using the electrocoagulation method will be very useful if it can be applied to home-based batik industry entrepreneurs. There are still many entrepreneurs who find it difficult to process their wastewater following government policies regarding the quality standards of wastewater before being disposed to the environment. (*)
Author: Aken Puti Wanguyun
Details of the research available at:
http://www.envirobiotechjournals.com/article_abstract.php?aid=9683&iid=276&jid=3