Various pulping methods, such as Kraft, sulfite, and soda, have been adopted to produce cellulose from lignocelluloses. Pulping methods such as semi-chemical, sodium hydroxide and sodium sulfide, and organosolv and sulfite acid have been employed in the time past. However, the chemical method has been used extensively to isolate cellulose from plant biomass, and the realization of the direct relationship between the pulping chemicals and the characteristics of the produced cellulose has triggered more research on the use of different chemicals and chemical mixtures for the production of cellulose. Sodium hydroxide solution is mainly used for delignify cation and cellulose extraction during pulping.
However, the low lignin removal and carbohydrate peeling effect of sodium hydroxide pulping are some of its drawbacks. To enhance the properties of cellulose, sodium hydroxide pulping has been modified by adding some other chemicals. Adding ethanol to soda pulping has been reported to increase the lignin removal and yield and produced a better result than using kraft pulping chemicals in ethanol production. The search for chemicals or chemical mixtures that could positively influence the properties of cellulose has been pivotal in various research on chemical pulping. Modifying the soda pulping method could produce cellulose with better properties than cellulose produced with either soda or soda anthraquinone pulping chemical. Ethanol and anthraquinone have been combined singly with soda, but there needs to be more information on the use of soda, ethanol and anthraquinone mixture for cellulose production.
In this study, instrumental investigation using scanning electron microscopy, ImageJ software, X-ray diffraction, Fourier Transform Infrared, and thermogravimetric analysis was carried out to elucidate surface morphology, fiber diameter, crystallinity, the functional group (with FTIR-derived crystallinity), and thermal stability of cellulose produced from pineapple leaf and siam weed (chromolaena odorata) stem as influenced by sodium hydroxide-anthraquinone-ethanol and sodium hydroxide-anthraquinone pulping chemicals.
Anthraquinone addition
This study developed mixtures of sodium hydroxide, ethanol, and anthraquinone as new pulping chemicals to produce alpha-cellulose from pineapple leaves and Saim weed stem s.
The addition of anthraquinone was noticed to influence the pulp yield positively and the FTIR of all the cellulose has the characteristic cellulose bands. Calculated Total crystallinity index (TCI), (Lateral order index) LOI., and Hydrogen bond intensity (HBI) from the FTIR presented PLSHAEC (68.38 %) and SWSHQEC (67.74 %) having high crystallinity. From the XRD, the 2θ by all the materials is attributed to cellulose I diffraction, and the crystallinity index aligned with FTIR determined crystallinity. The determined particle average diameter using ImageJ software showed that PLSHQEC 3.00 had the smallest value, followed by 5.17 for PLSHQC, 5.32 for SWSHQEC, and 6.03 for SWSHQEC. From the thermal analysis, the onset of the degradation of all the cellulose occurred at different temperatures: 247.10 C (SWSHQEC). The increase in yield, higher crystallinity index, and smaller fiber diameter of the cellulose demonstrated anthraquinone as a better additive to produce quality cellulose that would undergo easy chemical modification.
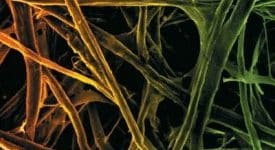
The fiber micrograph of the celluloses is presented in Figure 1. The influence of the pulping chemicals was evident from the images. The images of cellulose obtained with soda, ethanol, nd anthraquinone mixture look brighter, smoother, and shining than cellulose obtained with only soda and ethanol pulping chemicals. Ethanol addition to soda chemical can improve lignin selectivity towards degradation and dissolution, thereby reducing the impurity on the fiber surface. However, it is worth noting that lignin dissolution and degradation are enhanced by anthraquinone, which also prevents redeposition of lignin and other impurities (wax, pectin, extractives, hemicelluloses) on cellulose fiber surface. The overlay of long, bundle, firmly glued, and cloudy fiber was more conspicuous in SWSHQC than PLSHQC, testifying that non-cellulose compounds are still present in the fiber. The presence of silica in the EDX spectra of SWSHQC revealed the existence of non-cellulose materials. The removal of that non-cellulosic impurity allowed the proper interaction between the pulping chemical and fiber, leading to defibrillation, causing the PLSHQEC and SWSHQEC to appear as fiber strands instead of a bundle like the SWSHQC.
Conclusion
The proximate results of the raw materials (moisture content of 5 % siam weed and 6 % Pineapple), (ash content of 6.5 % siam weed and 9.50 % pineapple), (lignin contents of 21.40 % siam weed and 27.57 % pineapple), alpha-cellulose of 59.89 % pineapple and 56.08 % of siam weed) suggest them as good pulping candidates for cellulose production. Adding anthraquinone to sodium hydroxide-ethanol pulping chemical has proved to be a better alternative to sodium hydroxide-ethanol. It produced celluloses with a higher crystallinity index and fiber with a larger surface area due to the reduced fiber diameter determined by the ImageJ software.
The celluloses have higher thermal stability than those produced with sodium hydroxide and ethanol. The prominent peaks expected of cellulose are identifiable in the spectra of all the celluloses. The SEM analysis that showed the smaller morphological images of the cellulose by adding ethanol collaborated with the ImageJ and the crystallinity results. The functionalization reaction of those celluloses produced by ethanol addition would be faster and easier because of their larger surfaced area. Because of their crystallinity properties, the cellulose materials could be useful in thermal and strength demanding products. Also, the particle size of the materials could make the materials that can easily be modified to produce various cellulose derivatives.
Author: Prof. Mochamad Zakki Fahmi, S.Si., M.Si., Ph.D.
Link: https://journal.nsps.org.ng/index.php/jnsps/article/view/2033